Introduction:
Oxidative phosphorylation is an efficient method of synthesizing large amount of energy in the form of ATP atp molecules. This process involves inter-molecular exchange of electrons, thus, generating a chemical reactions which allows ATP formation. ETC is the most essential part of Oxidative phosphorylation that produces more ATP then any other part of cellular respiration.
Oxidative phosphorylation is defined as the oxidation of substrate by oxygen and phosphorylation of ADP to ATP via electron transport by cycle(ETC).
Mechanism:
Oxidative phosphorylation can be explained by the following hypotheses:
1) Chemical coupling hypothesis: This hypothesis was given by Edward Slater. According to him, during the transfer complex iv of electron in respiratory chain, a series of phosphorylated high-energy intermediates are formed; these intermediates are further utilized in ATP generation. These reactions are similar to substrate-level phosphorylation occurring in glycolysis or citric acid cycle. This hypothesis cannot be proven practically because none of the high-energy intermembrane space have been successfully isolated till now complex ii.
2) Conformational coupling hypothesis: As per this hypothesis, the cristae of mitochondria go through conformational changes which reflect the changes in the different components of the electron chain to one another these conformational changes also signify the formation of high energy state
3) Chemiosmotic hypothesis: This hypothesis was proposed by peter Mitchell. This hypothesis explains transport of electrons are passed through the ETC which is utilized for generating ATP from ADP +Pi. It is now widely accepted and can be exemplified with an energy stored battery separated by positive and negative charges.
Proton gradient:
The inner membrane of mitochondria is impermeable to protons (H+) and hydroxyl ion (0H-). So, the transport of electron via ETC is coupled with translocation of protons (H+ ) across the inner mitochondrial membrane (coupling membrane)from the matrix to the inter-membrane space. This builds-up electrochemical or proton gradient which is involved in ATP synthesis from ADP and Pi.
The protons that accumulate on the inter-membrane space re-enter the mitochondrial matrix leading to the synthesis of ATP.
Enzyme System for ATP Synthesis: ATP synthase, present in the complex V, utilises the proton gradient for the synthesis of ATP. this enzyme is also known as ATPase, since it can hydrolyse ATP to ADP and Pi. ATP synthese is a complex enzyme and consists of two functional subunits namely F1 and F0.its structure is comparable with ‘lollipops’.
Electron Transport Chain (ETC)/Respiratory Chain:-
The electron transport chain is the final common pathway of biological oxidation and is a system of proteins and organic molecule, therefore also known as electron transport system. The reaction site of ETC is inner membrane of the mitochondria. A chain of redox reactions passes the electrons from one membrane of the transport chain to another. These reactions liberate energy which is captured as a proton gradient and then utilised for ATP formation via chemiosmosis. The ETC and chemiosmosis together constitute the oxidative phosphorylation.
ETC consists of a series of membrane bound proteins and organic molecules, most of them arranged in four large complexes. In prokaryotes, the components of ETC are present in the plasma membrane, while in eukaryotes various copies of ETC components are found in the inner mitochondrial membrane.
In ETC, the electrons travel through the chain, passing from a higher to a lower energy level by moving through electron-rich to hydrogen ions electron-deficit molecules. These downhill electron transfers liberate energy which is utilised by the protein complexes for driving the protons from mitochondrial matrix to inter-membrane space, thus forming a proton gradient.
Components:-
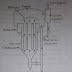
Most constituents of the respiratory chain are embedded in the inner mitochondrial membrane having infoldings called cristae. The surface area of inner mitochondrial membrane having infoldings called cristae. The surface area of inner mitochondrial membrane is increased by cristae. All the five components or carriers of ETC are arranged sequentially, thus transfer electrons from a given substrate to ultimately proton and oxygen (o) to from H2O. the five components or carriers of ETC are:
1) Nicotinamide nucleotides: the two important co-enzymes derived from niacin (vitamin) are NAD+ and NADP +, in which NAD+ is involved more actively in ETC. In the presence of enzyme dehydrogenases, the NAD+ is reduce to NADH + H+ and the substrate is oxidized simultaneously by removing two hydrogen atoms. Glyceraldehyde-3 phosphate, pyruvate, isocitrate, and malate may be the given substrates.
NADPH+H+ generated by the NADP+ depended dehydrogenase is not preferred as a substrate for ETC. NADPH is more effectively used in anabolic reactions such as fatty acid synthesis, cholesterol synthesis, etc.
2) Flavoproteins: The NADH dehydrogenase (NADH-Coenzyme Q reductase) enzyme is a flavoprotein. Its prosthetic group is the complex iii coenzyme FMN which produce FMNH2 by accepting two electrons and a proton.
NADH dehydrogenase is a complex enzyme and is found linked with Non-Heme Iron Proteins (NHI) or iron sulfur (FeS) proteins.
An enzyme succinate dehydrogenase ( Succinate Co-Q reductase) present in inner mitochondrial membrane is also a flavoprotein with FAD as a coenzyme. FAD accepts two hydrogen atoms from succinate forming FADH2 and fumarate.
3) Iron Sulphur Proteins: The FeS proteins either exists in the oxidized (Fe+3) or reduce (Fe+2) form. A FeS protein transfers electrons from FMN to coenzyme-Q ; while some FeS proteins molecular oxygen link with cytochrome b and cytochrome c1 and transport electrons.
4) Coenzyme Q : Coenzyme Q (or ubiquinone) is derived from quinine and has a variable isoprenoid side chain. It is a lipophilic electron carrier with electron acceptors from either FMNH2 ( produced in the ETC by NADH dehydrogenase) or FADH2 (produce outside the ETC by succinate dehydrogenase).
5) Cytochromes: These are conjugated proteins with a haeme group. Haeme consist of a porphyrin ring with iron atom. The haeme group of cytochromes differ from that found in the structure of haemoglobin and myoglobin nadh and fadh2.
The iron of these haeme groups undergoes continuous oxidation (Fe3+) and reduction (Fe2+), thus transport electron in the ETC. Unlike this, the haeme of haemoglobin and myoglobin always has the ferrous (Fe2+) state of iron.
Initially, three cytochromes were discovered in the mammalian mitochondria. They are named a, b, and c on the basic of the type of haeme present and the respective absorption spectrum. Later cytochromes c1, b1, b2, a3, etc., were also discovered.
Transport of electrons occurs from coenzyme Q to cytochromes in the order of b, c1, c, a, and a3. The reversible oxidation-reduction of haeme iron (Fe2+ --- Fe3+) in cytochromes makes them an effective electron carrier in ETC.
Components and reactions of the electron transport chain are diagrammatically represent in below picture.

















No comments:
Post a Comment